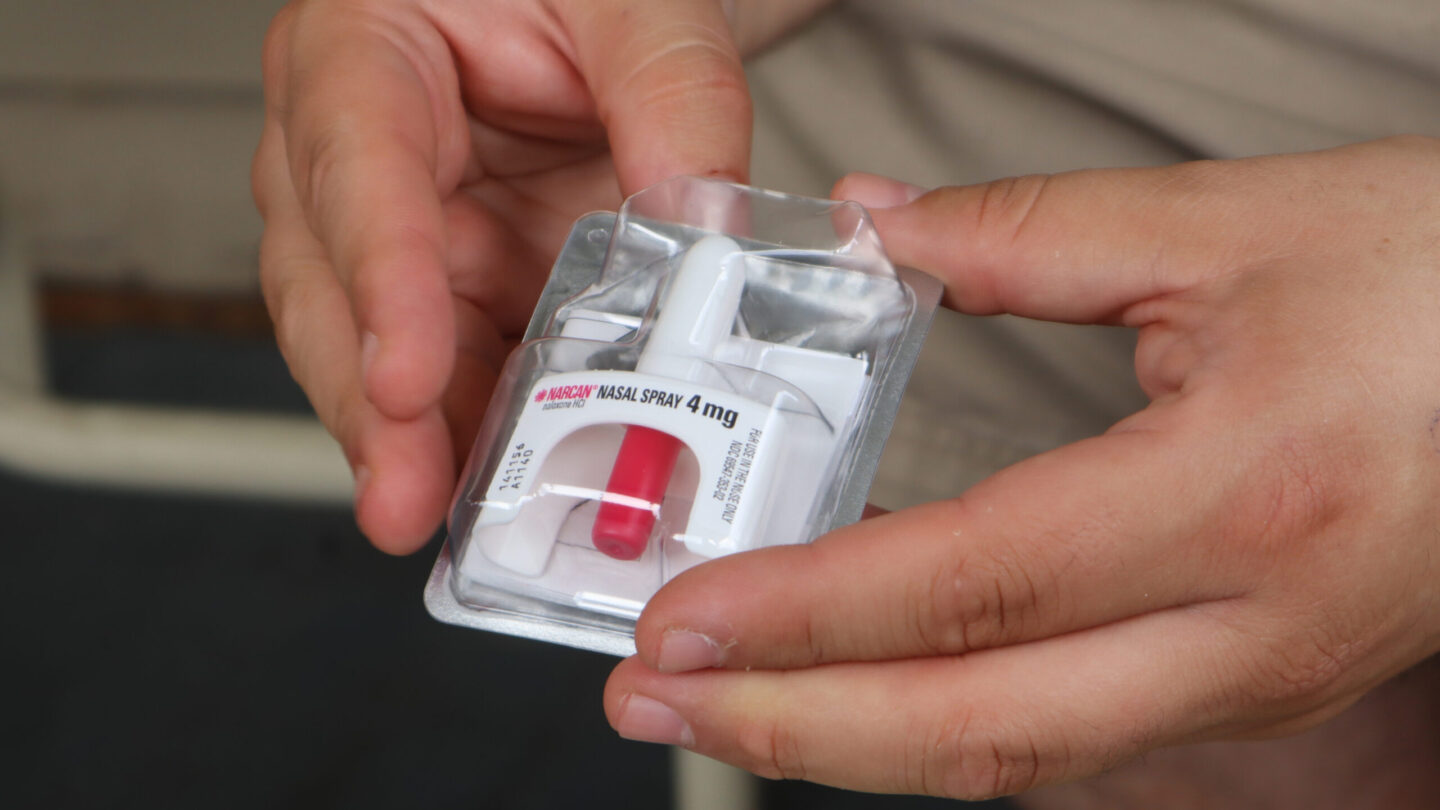
The overdose-reversing drug Narcan could soon be available to buy over the counter without a prescription, the Food and Drug Administration announced Wednesday.
The FDA’s approval of the nasal spray Narcan — the brand name for the drug naloxone — means the medication could be more widely available across the U.S. as the country continues to grapple with an opioid epidemic.
“Today’s action paves the way for the life-saving medication to reverse an opioid overdose to be sold directly to consumers in places like drug stores, convenience stores, grocery stores and gas stations, as well as online,” the FDA said in a statement.
It could take months before Narcan becomes available over-the-counter and the cost would be determined by the manufacturer, the administration added.
The specific dose approved for over-the-counter sales is the 4 milligram (mg) naloxone hydrochloride nasal spray. Other formulations and dosages of the drug would still require a prescription, the FDA said.
The administration first approved Narcan nasal spray in 2015 as a prescription drug.
9(MDAxODM0MDY4MDEyMTY4NDA3MzI3YjkzMw004))